60
AFC
Purification of Monoclonal Antibodies
Typically antibodies are captured at near neutral pH and
eluted using acidic conditions. The clarified feedstock is
loaded onto the column at a neutral pH. After sufficient
washing with the loading buffer, the antibody is eluted
at low pH. However, the physicochemical properties of
different mAbs are varying depending on the expression
system and antibody subclass. Therefore a generic method
needs to be optimized for each individual target in order to
establish conditions that will bind the highest amount of
the target molecule in the shortest time and elute it with the
highest purity. For initial scouting of method parameters
we recommend using pre-packed ToyoScreen columns or
robotic high throughput screening devices with ToyoScreen
RoboColumns.
Suitable load/wash buffers are 20-100mmol/L sodiumphos-
phate, 150 mmol/L NaCl, pH 7.2 - 7.5 or 100 mmol/L Tris-HCl,
150 mmol/L NaCl, pH 7.2 - 7.5. Washing at reduced pH (e.g.
pH 6) might further improve host cell protein reduction.
Suitable elution buffers are 100 mmol/L citrate, 100 mmol/L
acetate, or 100 mmol/L glycine-HCl. The pH shift required
for mAb elution depends on the particular mAb and ranges
from pH 3.0 to 4.5. For cleaning and sanitization the use of
0.1 to 0.5 molar NaOH is recommended. Depending on the
origin and subclass of the antibody, contact time, concen-
tration, and frequency of CIP cycles the conditions should
be optimized.
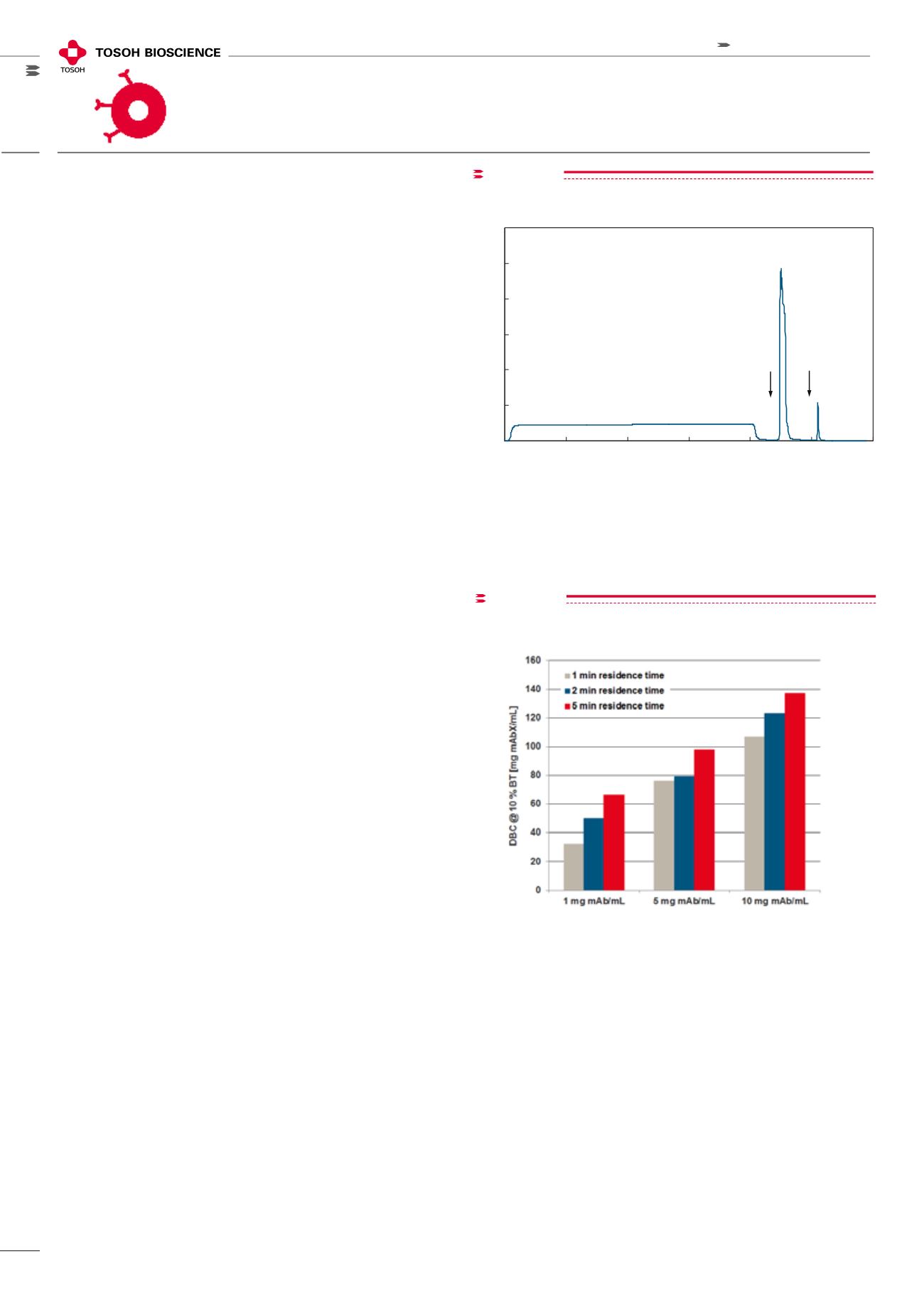
TOYOPEARL AF-rProtein A HC-650F was used for the puri-
fication of a monoclonal antibody from CHO cell culture
supernatant with a concentration of 1.0 g/L (Figure 10) at
5 minutes residence time in a 5 cm bed height column. As
can be seen from the chromatogram, tailing is minimal on
the elution peak and the eluted mAb is > 95% pure by SEC.
Figure 11 shows the binding capacities for the capturing of
a therapeutic monoclonal IgG1 spiked at different concen-
trations into CHO cell culture fluid. The binding capacity
of TOYOPEARL AF-rProtein A HC-650F for this specific
antibody is increasing dramatically with increasing feed
concentrations. Furthermore, when applying a feed concen-
tration of 10 mg mAb/mL a capacity of more than 100 mg
mAb/mL resin was even reached at 1 min. residence time.
ToyoScreen prepacked columns for process
development
ToyoScreen columns packed with the TOYOPEARL
AF-rProtein A resins are available in 1 mL and 5 mL resin
volumes. ToyoScreen columns provide a convenient way to
perform early resin screening for both target retention and
recovery. Multiple columns can be connected in series for
additional capacity. ToyoScreen RoboColumns are minia-
turized chromatographic columns for operation
with a robotic liquid handling system, such as the Freedom
EVO
®
from TECAN. This approach allows automated
highthroughput, small-scale biochromatographic separa-
tions of protein samples by running up to eight individual
columns simultaneously. ToyoScreen RoboColumns packed
with TOYOPEARL Protein A resins are available with 200 μL
and 600 μL resin volumes.
Protein a Affinity
chromatography
figure 10
0
500
1,000
1,500
2,000
2,500
3,000
0
50
100
150
200
250
300
Elution time (minutes)
Detectore response (mAU)
Elution buffer
CIP (0.1N-NaOH)
Resin:
Experimental TOYOPEARL Protein A
Column size:
5 mm ID × 5.0 cm
Flow rate:
0.2 mL/min
Sample:
40 mL of CHO cell culture,
containing 1.0 mg/mL humanized IgG
1
Binding buffer: 20 mmol/L sodium phosphate containing
0.15 mol/L NaCl, pH 7.4
Elution buffer:
0.1 mol/L citrate, pH 3.0
Resin: TOYOPEARL Protein A; Column size: 5 mm ID × 5.0 cm L; Mobile
phase: Buffer A: 20 mmol/L sodium phosphate containing 0.15 mol/L NaCl,
pH 7.4, Buffer B: 0.1 mol/L citrate, pH 3.0; Flow rate: 61 cm/h (0.2 mL/min);
Residence time: 5 min; Sample: 40 mL of CHO cell culture, containing 1.0 g/L
humanized IgG
1
PURIFICATION OF MONOCLONAL ANTIBODY
Column: TOYOPEARL AF-rProtein A HC-650F (6.6 mm ID × 2 cm L)
Mobile phase: 100 mmol/L sodium phosphate pH 6.5;
Residence time: 1, 2, 5 min; Detection: UV @ 280 nm
Sample: monoclonal antibody mAbX @ 1, 5, 10 g/L in mobile phase
DBC measured at 10 % breakthrough
figure 11
DBC for A specific mAb at various loads and velocities


