46
HIC
The retention and selectivity of protein standards on
TOYOPEARL HIC resins using the ToyoScreen process
development columns are shown in Figure 3.
Influence of salt type
In addition to the hydrophobicity of the ligand, the selec-
tivity in HIC is influenced by the eluent salt type. Figure 4
demonstrates the effect of salt type on the resolution factor
of different protein pairs.
The Hofmeister lyotropic salt series shown in Figure 5
ranks anions and cations by their ability to promote protein
precipitation. Ions on the left are referred to as “lyotropic”
while the ions on the right are called “chaotropic”.
Lyotropic salts will precipitate or “salt out” proteins at
high salt concentrations due to increased hydrophobic
interaction, while chaotropic salts will promote protein
denaturation at high salt concentrations. Figure 5 indicates
that different salt systems may generate a variety of adsorp-
tion and desorption selectivities for each resin. This feature
of HIC provides an additional parameter for the optimiza-
tion of a process step.
hydrophobic interaction
chromatography
Pore size (nm) 5 12,5 40-50 75 100 >100 >170
Product name
TOYOPEARL 40 50
55 60 65
75
80
HW-type
TSKgel
G1000 G2000 G4000 - G5000 G6000 -
PW-type
Methacrylic base beads available for HIC
figure 6
figure 5
For anions
SO
4
2-
> HPO
4
2-
> CH
3
COO
-
> halide > NO
3
-
> CIO
4
-
> SCN
-
For cations
(CH
3
)
4
N
+
> NH
4
+
> K
+
> Na
+
> Cs
+
> Li
+
> Mg
2+
> Ca
2+
> Ba
2+
Ammonium sulfate and sodium sulfate are the most commonly
used salts in HIC. NaCl is often used as well.
Hofmeister Lyotropic Salt Series
figure 4
Influence of salt-type on resolution
100
50
0
Resolution Factor (Rs)
Cytochrome c/Conalbumin
Conalbumin/
β
-Glucosidase
Na
3
Citrate
Na
2
SO
4
(NH
4
)
2
SO
4
NaCl
NH
4
SCN
Na
3
Citrate
Na
2
SO
4
(NH
4
)
2
SO
4
NaCl
NH
4
SCN
Chromatography on a Toyopearl Butyl-substituted support
Column Dimensions: 4.1mm ID x 4cm
Elution: Linear gradient, 20min, 1.0mol/L to 0mol/L of
indicated salt in 20mmol/L phosphate buffer
(pH7.0). Flow rate, 1mL/min
Detector: UV @ 280nm
J. Fausnaugh, L. Kennedy and F. Regnier, J. Chromatography 317, 141 (1984)
Chromatography on a TOYOPEARL Butyl-substituted su port
Column dimensions: 4.1 mm ID x 4 cm L
Mobile phase: Linear gradient, 20 min, 1.0 mol/L to 0 mol/L of
indicated salt in 20 mmol/L phosphate buffer (pH 7.0);
Flow rate, 1 mL/min; Detector: UV @ 280 nm
J. Fausnaugh, L. Kennedy and F. Regnier, J. Chromatography
317, 141 (1984)
Influence of salt-type on resolution
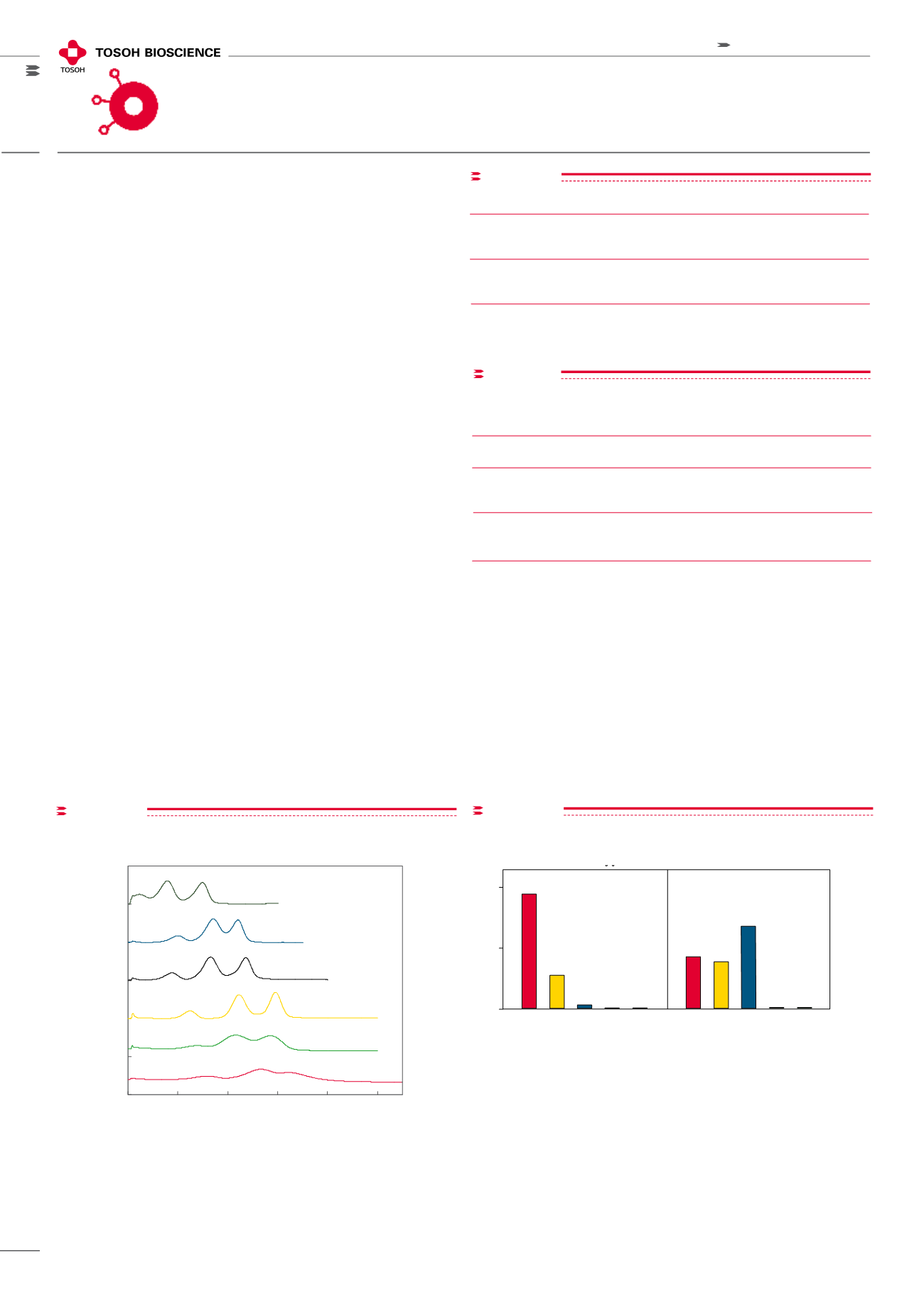
figure 3
0
50
100
250
200
250
300
0
10
20
30
40
50
Elution Time (min)
UV @ 280 nm (mAU)
Column: ToyoScreen (1mL)
Eluent A: 0.1mol/L Phosphate Buffer +1.8mol/L Sodium Sulfate (pH7.0)
Eluent B: 0.1mol/L Phosphate Buffer (pH7.0)
Gradient: 30min linear gradient from A to B
Flow Rate: 1mL/min
Inj. Vol.: 50µL
Detector: UV @ 280nm
Samples: Ribonuclease A, Lysozyme,
α
-Chymotrypsinogen, 1mg/mL
Screening of Toyopearl HIC resins - Standard Proteins
Ether-650M
PPG-600M
Phenyl-650M
Butyl-650M
SuperButyl-550C
Hexyl-650C
Column: ToyoScreen (1 mL)
Mobile phase A: 0.1 mol/L phosphate buffer + 1.8 mol/L sodium sulfate (pH
7.0); Eluent B: 0.1 mol/L phosphate buffer (pH 7.0);
Gradient: 30 min linear gradient from A to B; Flow rate: 1 mL/min; Inj.
vol.: 50 µL; Detector: UV @ 280 nm; Samples: Ribonuclease A, Lysozyme,
a
-Chymotrypsinogen, 1 g/L
Screening of TOYOPEARL HIC r i s - Stan ard Proteins


