18
IEC
TOYOPEARL Ion Exchange Chromatography
resins
Ion Exchange Chromatography (IEC) is the most common
liquid chromatographic method used in manufacturing
therapeutic proteins. Due to the high dynamic binding
capacities of ion exchange resins relative to those of the
other chromatographic modes, it is the chromatographic
technique selected by many developers for the capture or
concentration step. Tosoh Bioscience offers a broad range
of products for ion exchange applications.
How does IEC work?
IEC is based on the binding of proteins to positively or
negatively charged groups which are immobilized on a
stationary phase and which are in equilibrium with free
counter ions in the mobile phase. In the process of adsorp-
tion, the mobile phase counter ions are exchanged by the
protein solute. The binding of proteins to the ion exchange
matrix predominantly occurs via charged amino acid
residues located at the surface of the protein molecule.
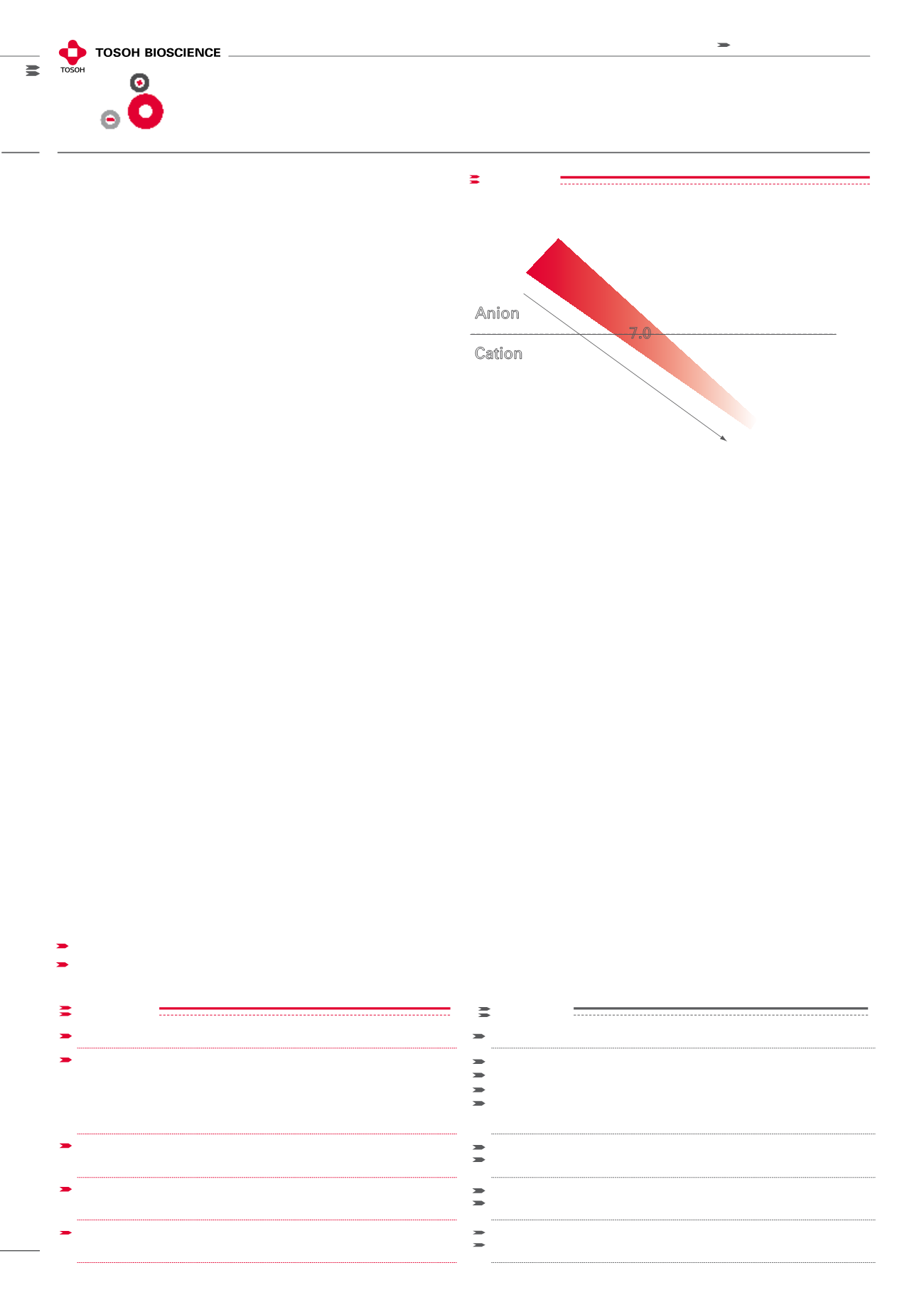
The development of optimum chromatographic system
conditions requires knowledge of both the protein’s pI and
the pKa of the ion exchange media. A binding buffer pH is
selected between the pI of the target and the ion exchanger’s
pKa (Figure 1). This ensures that the protein is in the opposite
charge state compared to the ion exchange media. When
possible, the pH is also optimized to effect the highest solu-
bility of the target protein. Higher protein solubilities make
more efficient use of the overall ion exchange capacity of
the resin. A salt is selected as the source of counter ions in
the mobile phase and elution occurs as the salt strength
is increased to a higher concentration than the target’s
binding salt conditions.
Ion exchange groups available
TOYOPEARL and TSKgel PW-type IEC resins are available
with six different ion exchange groups as shown in Table I:
3 for anion exchange – Q, QAE, DEAE
3 for cation exchange – S, SP, CM
Pore sizes
Tosoh Bioscience offers a range of pore sizes for IEC resins
originating from our size exclusion chromatography base
resins. Four different mean pore diameters are used for the
current ion exchange resins: 100 nm, 75 nm, 50 nm, and 25
nm (Table II). Depending on the kind of ligand attachment,
the effective pore size of the resulting IEC resin is smaller
than the pore size of the base bead. When network ligand
technology is applied the accessible pore size is varying
with pH and salt concentration, therefore all pore sizes
mentioned here are those of the respective TOYOPEARL
HW or TSKgel base resin.
Higher accessible surface area - more capacity
A bead with a small pore size has theoretically more surface
area than the same size bead with a larger pore. Figure 2
shows insulin binding capacity on six different pore size
beads. As the pore size increases to the point where the
insulin has maximal access to the internal surface area the
insulin capacity increases.
Ion exchange
chromatography
pK
a
values for Ion Exchange groups
Q, SuperQ, QAE pK
a
= 12.2
CM pK
a
= 4.7*
*pK
a
Toyopearl GigaCap CM-650M = 3.6
S, SP pK
a
= 1.2
DEAE pK
a
= 11.5
Cation
Anion
7.0
Decreasing resin pK
a
pK
a
values for ion exchange groups
figure 1
features
Benefits
porous, hydrophilic polymer based resin
suitable for laboratory scale and process chromatography
chemical stability
autoclavable at 121 °C
temperature range 4 - 60 °C
pH range 2-13, can be regenerated with acid or base
compatible with organic sovents
column bed stability
constant packing volume over a wide range of salt
concentrations
mechanical stability
excellent flow characteristics in large
industrial columns
continous selectivity
easy scale-up from TSKgel IEC columns
high yields of biologically active proteins


