Hydrophobic Interaction Chromatography
Hydrophobic Interaction Chromatography (HIC) is a powerful tool for the process purification of biomolecules. The technique utilizes the accessible hydrophobic regions located on protein surfaces and their interactions with a weakly hydrophobic stationary phase. Proteins and other molecules with hydrophobic surfaces are attracted to the hydrophobic ligands of HIC resins by employing an aqueous high salt mobile phase. The salt conditions contribute to a lyotropic effect which allows the proteins to bind to a hydrophobic ligand. Proteins are eluted by decreasing the salt concentration. Most therapeutic targets are eluted in a low salt or a no salt buffer. Since HIC separations are done under mild eluting conditions, biological activity is typically retained.
Applications
HIC is an excellent complement to ion exchange and size exclusion chromatography, particularly when protein isoforms exist or when feedstock impurities are of similar isoelectric point or molecular weight. The selectivity differences exploited by HIC can also be used after affinity separations in which closely related proteins with similar recognition sites are not distinguishable by the affinity ligand.
TOYOPEARL HIC Resins
TOYOPEARL HIC resins are functionalized versions of the TOYOPEARL HW size exclusion resins and are therefore based on hydroxylated polymethacrylic polymer beads.
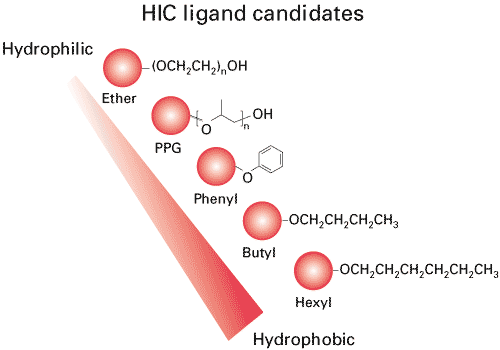
Tosoh Bioscience offers five HIC ligands featuring different degrees of hydrophobicity and selectivity. The hydrophobicity of HIC resins increases through the ligand series: Ether, PPG (polypropyleneglycol), Phenyl, Butyl, and Hexyl.
TOYOPEARL HIC Ligands
|
 |